Wastewater Treatment
Nitrogen Removal
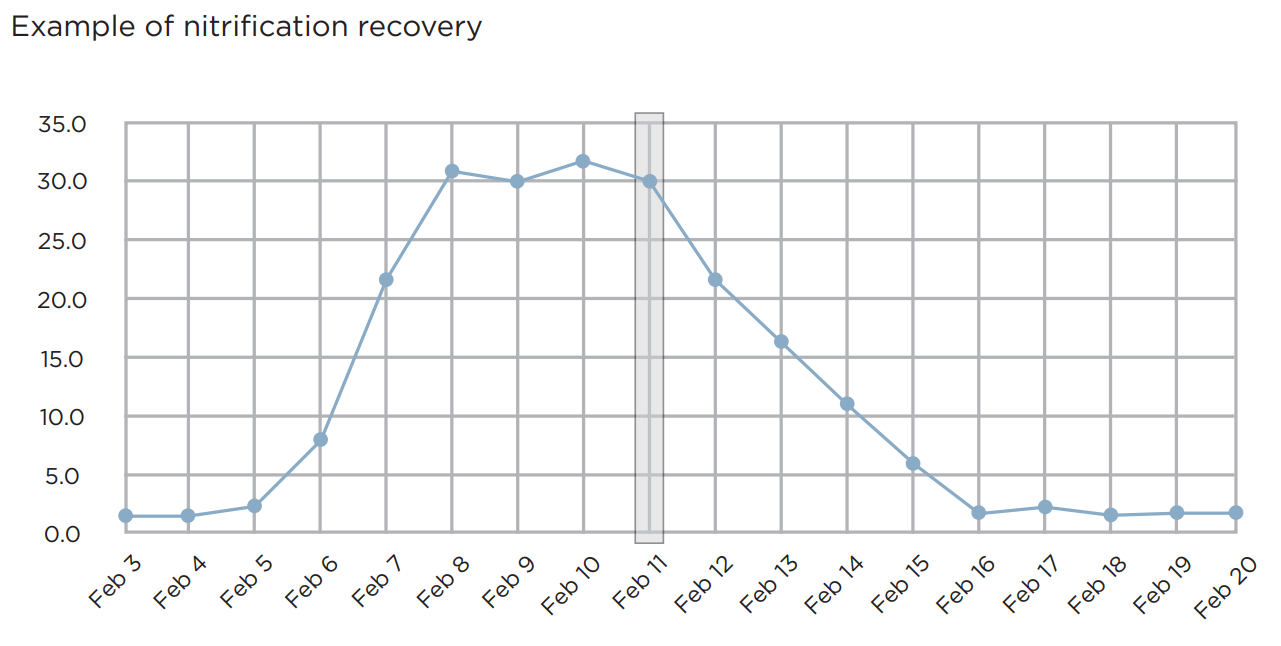
Full Recovery in 10 Days or Less
Nitrification processes can quickly decline from toxicity or cold conditions.
For the fastest recovery from such a decline and to remove ammonia we recommend SELECT-Nitrifiers® A & B, Nitrifiers and Ammonia Assimilators to quickly remove ammonia and restore nitrification.
This product is for use in aerobic wastewater treatment plants.
Learn more...
SELECT-Nitrifiers® Part A
SELECT-Nitrifiers® Part A
Use in conjunction with SELECT-Nitrifiers® Part B
SELECT-Nitrifiers® Part A contains high concentrations of autotrophic ammonia and nitrite-oxidising bacteria, such as Nitrospora, Nitrosomonas, Nitrospira and Nitrobacter as well as a micronutrient helpful to the growth and reproduction of nitrifying bacteria.
Full Recovery in 10 Days or Less

The Science Behind It
SELECT-Nitrifiers® Promote Fast Recovery
SELECT-Nitrifiers® makes use of the combination of Ammonia Assimilators and Nitrifiers for rapid ammonia removal. They contain high amounts of active nitrifying bacteria including Nitrosomonas, Nitrospira, Nitrobacter and heterotrophic nitrifiers at the highest concentration in the industry. They also contain powerful micronutrients to boost the growth and reproduction of the nitrifying bacteria.
The combination of these two products produces the fastest nitrification recovery. The biostimulants and hetrotrophic cultures in the SELECT-Nitrifiers® Part A Ammonia Assimilators work with the SELECT-Nitrifiers® Part B concentrations to produce the highest and fastest growth rate.
The SELECT-Nitrifiers® are specifically adapted to work in a range of wastewater conditions, taking into account the chemistry you currently use, any physical limitations of the plant and the present microorganism population.

Nitrite Lock in Industrial Plants
Nitrite Lock is an issue where nitrite oxidizing bacteria are unable to keep up with nitrite production by ammonia oxidizing bacteria. This leads to an accumulation of nitrite which can result in inhibition of ammonia removal. This rarely is experienced in municipal systems but can often occur in industries with high levels of incoming ammonia. Supporting a strong population of nitrifying bacteria can help prevent conditions like this from arising.
Applications
- Municipal Wastewater
- Industrial Wastewater
- Refineries
- Rendering Facilities
- Aquaculture
Benefits
- Rapid recovery from upset
- Improved nitrification
- Super concentrated formula
- Lower effluent ammonia
- Rapid start-up of new plants
- Creates healthier biomass
- Increases cell metabolism
Downloads
SELECT-Nitrifiers® Part B
SELECT-Nitrifiers® Part B
Use in conjunction with SELECT-Nitrifiers® Part A
SELECT-Nitrifiers® Part B contains heterotrophic nitrifying bacteria that utilise both carbon and a high fraction of nitrogen. In systems with high organic loading or low dissolved oxygen the Ammonia Assimilators perform the first step in removing ammonia from wastewater.
Full Recovery in 10 Days or Less

The Science Behind It
SELECT-Nitrifiers® Promote Fast Recovery
SELECT-Nitrifiers® makes use of the combination of Ammonia Assimilators and Nitrifiers for rapid ammonia removal. They contain high amounts of active nitrifying bacteria including Nitrosomonas, Nitrospira, Nitrobacter and heterotrophic nitrifiers at the highest concentration in the industry. They also contain powerful micronutrients to boost the growth and reproduction of the nitrifying bacteria.
The combination of these two products produces the fastest nitrification recovery. The biostimulants and hetrotrophic cultures in the SELECT-Nitrifiers® Part A Ammonia Assimilators work with the SELECT-Nitrifiers® Part B concentrations to produce the highest and fastest growth rate.
The SELECT-Nitrifiers® are specifically adapted to work in a range of wastewater conditions, taking into account the chemistry you currently use, any physical limitations of the plant and the present microorganism population.

Nitrite Lock in Industrial Plants
Nitrite Lock is an issue where nitrite oxidising bacteria are unable to keep up with nitrite production by ammonia oxidising bacteria. This leads to an accumulation of nitrite which can result in inhibition of ammonia removal. This rarely is experienced in municipal systems but can often occur in industries with high levels of incoming ammonia. Supporting a strong population of nitrifying bacteria can help prevent conditions like this from arising.
Applications
- Municipal Wastewater
- Industrial Wastewater
- Refineries
- Rendering Facilities
- Aquaculture
Benefits
- Rapid recovery from upset
- Improved nitrification
- Super concentrate formula
- Lower effluent ammonia
- Rapid start-up of new plants
- Creates healthier biomass
- Increases cell metabolism
Downloads
Why is Nitrifier Growth so Slow?
Why is Nitrifier Growth so Slow?
Nitrogen occurs in many forms in wastewater and undergoes numerous transformations. These transformations allow the conversion of ammonia-nitrogen to products that can more easily be removed. In the first step of nitrogen removal, nitrification, the oxygen demand of ammonia is reduced by converting it to nitrate. This is then followed by denitrification where the nitrate is converted to nitrogen gas which bubbles off back into the atmosphere.
Nitrification of Ammonia is a fragile process and must be controlled carefully.
Chemoautotrophic bacteria use CO2 as their carbon source and oxidation of non-organic material to generate cellular energy. The oxidation of inorganic material does not yield as much energy as the oxidation of organic carbon sources, as performed by heterotrophic bacteria, so nitrifiers have a very slow growth rate within the microbe community in wastewater plants.
Nitrifying bacteria are autotrophs, and use CO2 as the source of carbon for their cellular building material, so they do not contribute to the removal of BOD in the system. However, nitrifying bacteria are important to the system since decrease levels of ammonia to a concentration where heterotrophic bacteria are able to survive.
Although Nitrosomonas, Nitrobacter, and Nitrospira are commonly found in the soil and can easily wash into wastewater plants (WWTPs), many plants lose these bacteria because of environmental conditions, contamination with toxic compounds, low dissolved oxygen, or competition with other microorganisms.
Watch the 2019 Webinar on ammonia removal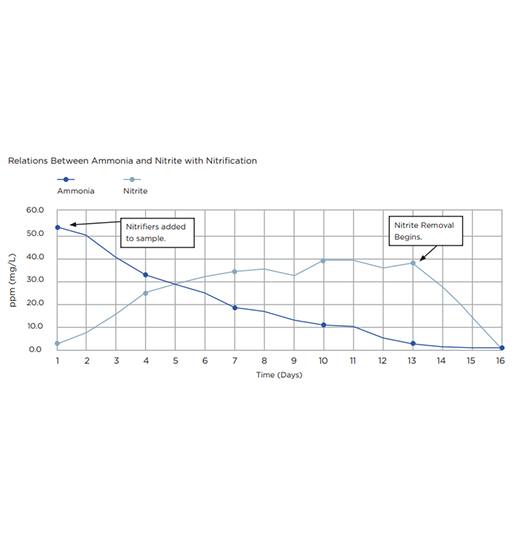
Seven Steps to Nitrification
Seven Steps to Nitrification
Factors Affecting Performance of Nitrifying Bacteria
1. Retention Time
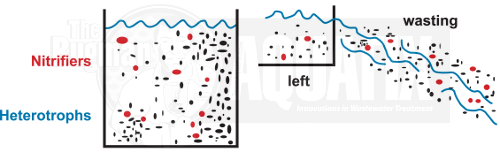
Inside the basin, the nitrifier populations are low, about 10% of the total bacterial population. Nitrifiers are slow growers, so if you waste heavily for a few days in a row or when temperatures are low, the entire population may be lost.
2. Temperature
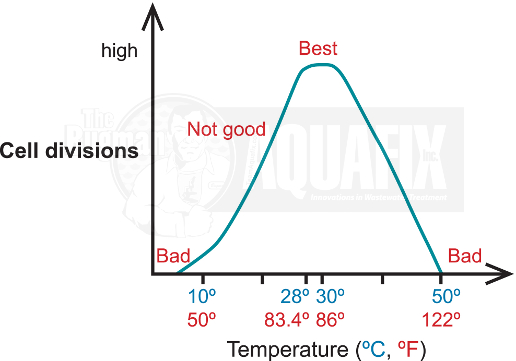
At temperatures less than 20°C (68°F), cellular metabolism slows down and fewer cells are nitrifying and dividing. Above 40°C (104°F), the proteins will be inactive and the cell membranes may break apart resulting in cell death.
3. Dissolved Oxygen
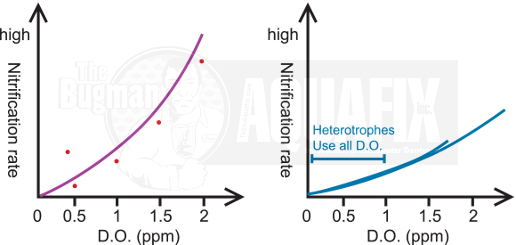
Heterotrophs have greater numbers than nitrifiers in wastewater and are more efficient at scavenging oxygen. In mixed populations with low D.O., the heterotrophs will be able to quickly use the available oxygen faster than the nitrifers since oxygen is also consumed in heterotrophic metabolism. It is a good idea to increase the D.O. levels with high incoming BOD or NH3. Remember: D.O. levels increase with colder temperatures and decrease in warmer temperatures.
4. Carbonate Alkalinity
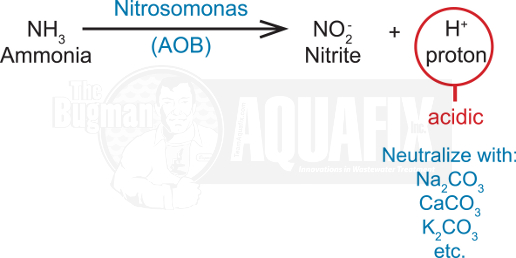
High rates of ammonia oxidation will acidify the environment and must be neutralized with additions of carbonate alkalinity (e.g., Na2CO3, CaCO3, or K2CO3) since 4 – 7 ppm alkalinity are used for every 1 ppm of ammonia. The biocatalyst in Nitrosomonas that carries out the first part of ammonia oxidation is inhibited (rendered inactive) by pH less than 7.
5. pH
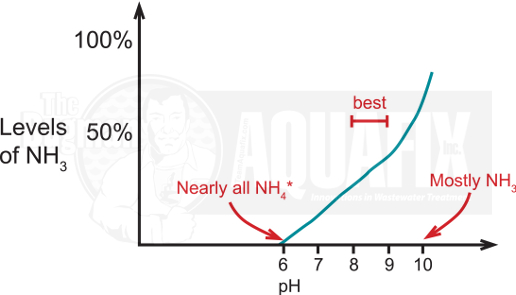
AOB only utilize ammonia not the ammonium ion. The levels of ammonia to ammonium vary depending on temperature and, more importantly, on pH. At lower pH values, most of the ammonia is in the ammonium form leaving you with a lot of nitrogen that the AOB cannot get rid of and conditions that would inactivate the ammonia-oxidizing biocatalyst. The ideal pH for ammonia oxidation is between pH 7-8.
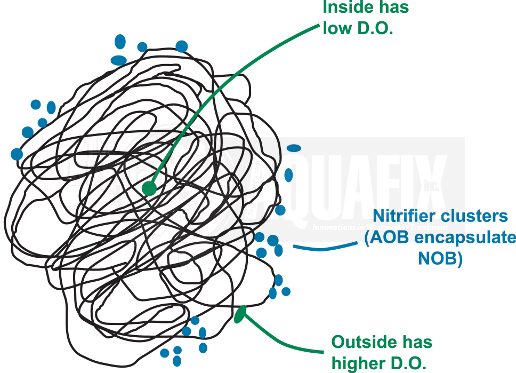
6. Floc Formation
Nitrifiers typically form aggregates of AOB and NOB along the edges of flocs made up of heterotrophic bacteria where the D.O. concentrations are high and they can still retain the protection of the biofilm. NH3 contains more energy per mol than NO2– so AOB may synthesize a capsule to encompass both cell types.
7. Toxicity
Heavy metals such as nickel, copper, zinc, cadmium and chromium can be toxic to nitrifiers. Exact levels of each are difficult to determine. Over chlorination can also cause toxicity. Also too high of ammonia (thousands of ppm) or a buildup of the intermediate nitrite can also cause toxicity. In short, nitrifiers are sensitive to toxicity, they are fickle and when temperatures get cold they are more subject to toxic effects of plant design, capacities, efficiency, and plant operation.